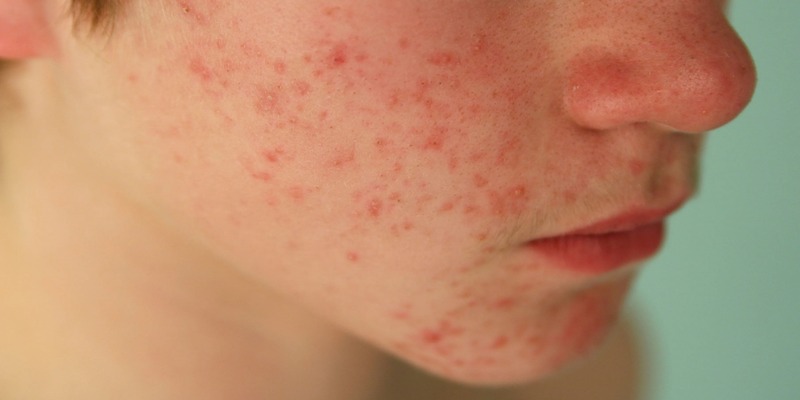
Most people who use Accutane have dry lips (90 percent), dry skin and itching (80 percent), and dry nasal pages with minor nosebleeds (80 percent). Muscle and joint discomfort only affect 15% of users.
Two to four weeks after beginning treatment is when these symptoms often emerge. The intensity of side effects may depend on the dosage that was provided. It's possible that people using smaller amounts won't have any side effects, while those taking greater doses could suffer a plethora of products, some of which might be rather severe.
Accutane Causes Serious Side Effects

Accutane's serious adverse effects are unusual. One study found that only 1/500 patients experienced serious side effects from the drug. There have been isolated reports of potentially fatal side effects, including elevated cerebral pressure and gastrointestinal issues.
The results of Accutane are long-lasting, even after treatment has been discontinued. Long-term visual and hearing loss may result from pressure on the brain. There have been isolated reports of irreversible vision loss and even death.
Long-term Accutane use is also associated with difficulties, including impotence, decreased libido, mood disorders, musculoskeletal troubles, and an increased risk of developing diabetes.
Pregnancy Complications Linked to Accutane
A black box warning stating the "very high risk" of "severe birth abnormalities" has been included on the Accutane label since 1985. Accutane should not be taken by women who are pregnant or who want to get pregnant, according to the drug's labeling. Isotretinoin has been linked to various birth abnormalities, including miscarriages and congenital malformations.
A defect is present at birth in the range of 3–5% of pregnancies. More than 35% of babies whose mothers used Accutane during pregnancy were born with abnormalities, and this was true even if the medicine was only taken for a short time.
Issues with newborns being born with underdeveloped eyes and hearing are also a problem. Heart malformations and cerebrospinal fluid accumulation are two more anomalies that are far less frequent but just as fatal.
Pregnancy and Isotretinoin
About half of all Accutane users are women of reproductive age. This raises serious concerns about the possibility of Accutane-related birth abnormalities. From 1982–2006, more than 2,000 pregnant women experienced pregnancies that ended in spontaneous or opted-for terminations.
Consequently, Roche initiated a program aimed at reducing the likelihood of pregnancy among its employee. Isotretinoin patients are mandated to participate in the iPLEDGE program. Women participating in the program must have monthly pregnancy tests at a medical facility.
Isotretinoin users must also enroll in the iPLEDGE program, even if they are men or women of reproductive age who cannot have children. Birth malformations caused by Accutane have decreased thanks to the iPLEDGE initiative, although they have not been eliminated.
Relation of Accutane to IBD and UC
Accutane has been associated with two gastrointestinal diseases in certain research: Crohn's disease and ulcerative colitis (UC). People who used isotretinoin were more likely to develop irritable bowel illnesses (IBD), which include Crohn's disease and ulcerative colitis, according to research published in the Journal of the American Academy of Dermatology in 2020.
However, the risk was minimal, and there was no statistically significant increase in risk for individuals who did not take isotretinoin. So far, there has been no link between Accutane and Crohn's disease established by the FDA in the United States. Instead, it cautions about stomach problems from using the drug.
Inflammatory Bowel Disease and Accutane (IBD)
According to research conducted at the University of North Carolina, isotretinoin users were shown to have a 1.68-fold increased risk of inflammatory bowel disease (IBD) compared to non-users. Patients who filled four or more prescriptions for Accutane had a rate 2.67 times higher than those who completed no medications.
A more recent meta-analysis published in the European Journal of Gastroenterology &'' Hepatology revealed no difference in the incidence of inflammatory bowel disease (IBD) between individuals who took and did not take isotretinoin.
Accutane use was not associated with an increased risk of inflammatory bowel disease or Crohn's disease. The risk of inflammatory bowel disease (IBD) is included on the most recent medication label for isotretinoin.
Accutane and Its Effects on Your Mind
Studies on the effects of Accutane on mental health conditions such as depression, psychosis, and suicidal ideation have shown conflicting findings. Whether or not the medicine causes individuals to be more susceptible to depression is still being investigated.
From 1982 through about the middle of 2000, the FDA received reports of 37 suicides, 110 hospitalizations for depression or suicidal conduct, and 284 episodes of depression that did not need hospitalization among patients using Accutane.
Roche, the drug's manufacturer, added a warning about isotretinoin's potential to cause mental health issues to Accutane's label in 1998. The Journal of the American Academy of Dermatology released research in 2017 showing little evidence of an increased risk of depression due to Accutane.